Whitefly-transmitted and Yellowing Viruses in Watermelon and Other Cucurbit Crops
A Photo Guide to Whitefly-transmitted and Yellowing Viruses in Watermelon and Other Cucurbit Crops
Whitefly-transmitted Viruses in Cucurbit Crops
Cucurbits, including watermelons, cantaloupes and other melons, cucumbers, pumpkins, and squashes, are important agricultural crops in many U.S. states. Many of these crops are grown in the field, while some, particularly cucumbers, may also be grown in enclosed structures, such as high tunnels and greenhouses. Florida, Texas, Georgia, and California lead the U.S. in watermelon production. However, watermelon is produced commercially on some level in many states, including throughout the Southeast. Other cucurbit crops, such as cucumbers, pumpkins, and squashes, are also produced commercially in these states and contribute to the agricultural economy of the region.
A variety of diseases, disorders, and pests may damage a crop during any given growing season, leading to reduced or poor-quality yields. Cucurbits, like all crops, can be infected by disease-causing organisms known as pathogens, including fungi, bacteria, and viruses. Viruses can cause many types of symptoms on plants, including stunting, leaf curling, necrosis or vine collapse, mottles or mosaics, bumpiness on leaves and fruit, and chlorosis (yellowing). In cucurbit crops, most of the viruses known to cause economically important diseases are transmitted by insects, and many induce foliar yellowing symptoms. These are known as “yellowing viruses.” Most yellowing viruses are transmitted by whiteflies, although at least one is transmitted by aphids. A few yellowing viruses have been present in the U.S. for many years, but within the past two decades, the number and distribution of these viruses has greatly expanded. This increase has largely coincided with an increase in the movement of plant material, including grafted transplants, which play an important role in the management of some diseases. Because transplants may remain symptomless up to 3 weeks after infection, infected transplants may unknowingly be moved among regions and lead to accidental introduction of viruses. In addition, yellowing viruses generally produce symptoms that mimic nutritional deficiencies, and this has historically caused virus infections to go undetected.
In addition to yellowing viruses, other whitefly-transmitted viruses can also lead to severe losses in cucurbit production. These viruses often occur in fields together with yellowing viruses. Mixed infections (where different viruses infect the same plant) can alter the appearance of symptoms, which makes field identification challenging. Many of the whitefly-transmitted viruses that originally emerged in a specific region of the U.S. have now been accidentally introduced to most other production regions. For example, cucurbit yellow stunting disorder virus (CYSDV) and cucurbit leaf crumple virus (CuLCrV) were established in California and Arizona but are now prevalent in the Southeastern U.S. Similarly, squash vein yellowing virus (SqVYV) was originally identified in Florida but is now found in California and Arizona. In both cases, movement of infected plant material is suspected as the source of introduction.
The two types of whitefly known to spread viruses among cucurbit crops in the U.S. are the sweetpotato whitefly (Bemisia tabaci) and the greenhouse whitefly (Trialeurodes vaporariorum). The sweetpotato whitefly (Figure 1A) is white, often with a pale-yellow body, and its wings are angled like the roof of a house, but with a distinct gap between the wings. In contrast, the greenhouse whitefly (Figure 2) is slightly larger than the sweetpotato whitefly, and its wings are more horizontal with only a slight angle (frequently overlapping one another), more like the shape of a fighter jet. Nymphs (immature whiteflies) of these species are also easy to differentiate. When viewed through a magnifying lens or microscope, sweetpotato whitefly nymphs are smooth (Figure 1B), whereas greenhouse whitefly nymphs are oval and have long, waxy, hair-like structures (Figure 2). Knowing the type of whitefly present in crops can help determine what viruses may be present.
As previously mentioned, watermelon and other cucurbit producers face a variety of challenges throughout the growing season. Diagnosis is the first step in disease management. Identifying the cause of a problem is necessary before appropriate management methods can be taken. This publication provides information on the yellowing viruses that can infect watermelon and other cucurbit crops as well as descriptions of the symptoms associated with the diseases caused by those viruses. Several plant pathology and entomology terms are used in these descriptions. These terms are formatted in bold and italics at their first mention within the text and are defined in the Glossary of Plant Pathology and Entomology Terms at the end of this publication. General management methods that can be used to prevent or reduce diseases caused by these viruses are described in the Management section.

Figure 1. Sweetpotato whitefly (Bemisia tabaci) adults (A) and nymphs (B). Photos: D. Riley, University of Georgia, Bugwood.org.

Figure 2. Greenhouse whitefly (Trialeurodes vaporariorum) adults and nymphs. Photo: W. Cranshaw, Colorado State University, Bugwood.org.
Viruses that Cause Yellowing in Watermelon and Other Cucurbit Crops
Whitefly-transmitted Viruses
Cucurbit yellow stunting disorder virus (CYSDV; genus Crinivirus) is transmitted by at least two biotypes of the sweetpotato whitefly. Biotype B is common in vegetable production regions in the U.S., whereas biotype Q is emerging in parts of the southeastern U.S. Whiteflies acquire CYSDV when feeding on infected plants and can retain and transmit the virus for 7 to 9 days without having to feed again on an infected plant. CYSDV can infect all major cucurbit crops, including watermelons, melons, squashes, pumpkins, and some wild cucurbits such as buffalo gourds (Cucurbita foetidissima). In addition to cucurbits, CYSDV can infect beans, lettuce, and numerous weeds (Wintermantel et al., 2009). In the U.S., CYSDV is known to infect cucurbit crops in Arizona, California, Florida, Georgia, and Texas, and has recently been reported in South Carolina (Kousik and Adkins, 2020).
Symptoms: CYSDV, like other criniviruses, has a long latent period, and symptoms will not appear until 3 weeks after plants become infected. As such, symptoms may appear well after whitefly populations have been reduced. Symptoms begin as a mottle but then develop into an extensive yellowing between the veins of leaves (Figures 3 and 4). Symptoms initially appear on older leaves while younger leaves remain green, but over time, symptoms spread down vines toward newer growth. CYSDV infection can limit plant vigor and reduce fruit sugar content, resulting in fruit that are often not marketable.
Management: clean transplants, timing of planting, vector management, and weed host management

Figure 3. Symptomatic melon leaves from plants infected with cucurbit yellow stunting disorder virus. Early stages of symptom development, a yellow-green mottle, are visible in 3A. More advanced stages of symptom development in which the entire leaf exhibits interveinal chlorosis with veins remaining green are visible in 3B. Photos: W. M. Wintermantel, USDA-ARS.

Figure 4. Mild foliar yellowing on a watermelon leaf caused by cucurbit yellow stunting disorder virus infection. Photo: W. M. Wintermantel, USDA-ARS.
Cucurbit chlorotic yellows virus (CCYV; genus Crinivirus), like CYSDV, is transmitted by at least two biotypes of the sweetpotato whitefly, biotypes B and Q. The virus is acquired when whiteflies feed on infected plants, and once acquired, whiteflies can transmit CCYV for a period of 11 to 14 days without having to feed again on an infected plant. CCYV can infect all cultivated cucurbits, including cucumbers, pumpkins (processing), melons, and watermelons. In addition to cucurbits, CCYV has been found to infect a number of other plants, including beets, lettuce, spinach, and some weeds (Okuda et al., 2010). CCYV was recently identified in California (Wintermantel et al., 2019); due to similarity in symptoms with other yellowing viruses, it may be present yet undetected in other areas of the U.S.
Symptoms: Symptoms on cucumbers, melons, watermelons, and some other cucurbits begin as chlorotic spots that progress to extensive yellowing of leaves, while leaf veins typically remain green. Symptoms initially appear on older leaves near the crown, but over time spread toward new growth, with the newest leaves often remaining green until late in disease development (Figure 5). CCYV infection can lead to reduced fruit sugar content and yield in watermelons and melons and reduced yield in cucumbers.
Management: clean transplants, timing of planting, vector management, and weed host management
Beet pseudoyellows virus (BPYV; genus Crinivirus) is transmitted by the greenhouse whitefly but not by the sweetpotato whitefly. Once the whitefly acquires the virus, it can transmit it to new plants for up to 6 days. BPYV is capable of infecting an unusually wide range of host plants, including members of the cucurbit family and a large number of ornamentals, small fruits, vegetables, and weeds. The virus is believed to be widely distributed throughout the world and is found in association with infestations of the greenhouse whitefly.
Symptoms: Like its close relatives, CYSDV and CCYV, symptoms of BPYV typically do not appear until 3 to 4 weeks after infection. On most cucurbits, symptoms begin as a mottle and develop into extensive interveinal chlorosis. These symptoms begin on older leaves or leaves near the crown of the plant but may progress down vines to younger leaves (Figure 6). Infected plants have reduced vigor and produce smaller fruit that is typically not marketable due to taste and sugar content.
Management: timing of planting, vector management, and weed host management

Figure 5. A melon field in California illustrates how yellowing begins near the crowns at the center of rows and progresses outward down vines. Most melon plants in this field are infected with both cucurbit yellow stunting disorder virus and cucurbit chlorotic yellows virus. Photo: W. M. Wintermantel, USDA-ARS.

Figure 6. Symptoms of beet pseudoyellows virus on pumpkin (Cucurbita maxima) showing yellowing of leaves near the crown with younger leaves remaining green. Photo: W. M. Wintermantel, USDA-ARS.
Cucurbit leaf crumple virus (CuLCrV; genus Begomovirus) is transmitted by the sweetpotato whitefly. The virus can be acquired during short feeding periods on infected plants, but efficient acquisition requires feeding for several hours. Unlike some of the yellowing viruses, once acquired, CuLCrV can be transmitted for the life of the whitefly. CuLCrV infects several cucurbits, but pumpkins, squashes, and watermelons appear to be particularly susceptible. A few cucurbit crops, including cucumbers and butternut squash, appear to have some level of resistance, and a source of resistance has been identified in melons (McCreight et al., 2008). Beans may also become infected with CuLCrV. In the U.S., CuLCrV is known to occur in Arizona, California, Florida, Georgia, South Carolina, and Texas.
Symptoms: The type and severity of symptoms can vary depending on the host and time of infection. Leaves of infected pumpkins, squashes, and watermelons are often thickened with distorted growth, and exhibit crumpling, curling, vein distortion, and a light green to yellow mottle (Figures 7 and 8). Infected plants may become stunted and distorted, and fruit from infected pumpkin or squash plants may be bumpy and mottled. Watermelon and muskmelon plants exhibiting symptoms, however, may recover 3 to 4 weeks after infection with no symptoms appearing on new growth; therefore, CuLCrV may have reduced impact on production of watermelons or muskmelons.
Management: resistance, site selection, clean transplants, and vector management

Figure 7. Symptoms on melon leaves caused by cucurbit leaf crumple virus infection, including leaf crumpling and yellow-green mottling. Photo: R. L. Gilbertson, University of California-Davis.

Figure 8. Crumpling and yellow-green mottling symptoms on watermelon leaves caused by cucurbit leaf crumple virus infection. Photo: M. R. Rojas, University of California-Davis.
Squash vein yellowing virus (SqVYV; genus Ipomovirus) is transmitted by the sweetpotato whitefly. Whiteflies that feed on and acquire SqVYV from infected hosts may be able to transmit the virus to new plants in as little as 30 minutes; however, whiteflies cannot retain the virus over long periods of time and lose the virus fairly quickly if removed from infected plants. Cucumbers, melons, squashes, and watermelons can become infected with SqVYV. The virus is most severe in watermelons. SqVYV causes the vines of infected watermelon plants to collapse and decline rapidly (Adkins et al., 2013). SqVYV is also known to infect various weeds in the cucurbit family, including balsam pear (Momordica charantia) and smell melon (Cucumis melo group Dudaim); these infected weed plants are symptomless but can serve as a source from which whiteflies can transmit the virus. In the U.S., SqVYV is known to occur in California, Florida, Georgia, and South Carolina.
Symptoms: In most commercially produced susceptible cucurbits, veins of infected leaves become yellow and may exhibit a netted appearance (Figure 9). In watermelons, infected vines initially exhibit vein yellowing, as seen in other cucurbit crops, but within a few days, petioles and then stems become necrotic, causing leaf and vine collapse, respectively (Figure 10). This collapse typically occurs near harvest. Fruit from infected watermelon plants are often not marketable due to flesh degradation, internal rind necrosis (Figure 11), and changes in acid and sugar content.
Management: clean transplants, vector management, weed host management, and post-harvest crop destruction

Figure 9. Vein yellowing in zucchini squash infected with squash vein yellowing virus. Photo: W. M. Wintermantel, USDA-ARS.

Figure 10. Leaf and vine collapse in a field of watermelon plants infected with squash vein yellowing virus. Photo: S. Adkins, USDA-ARS.
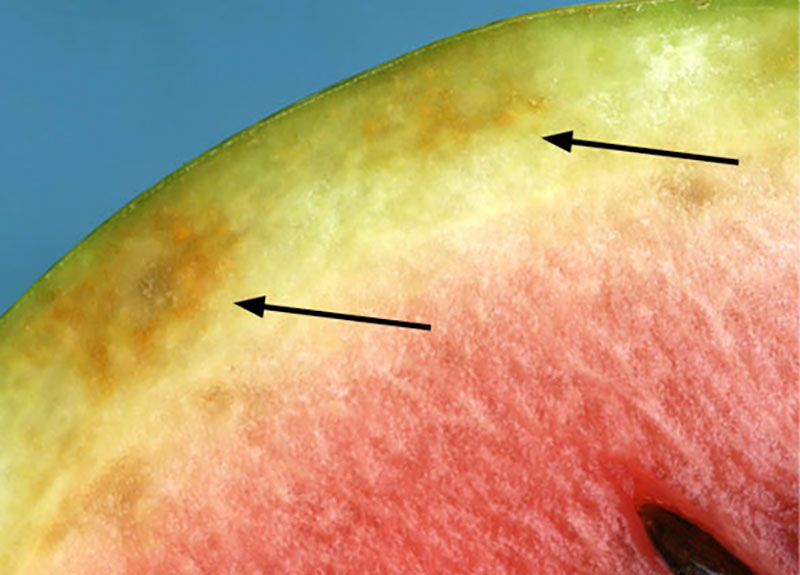
Figure 11. Internal rind necrosis in a watermelon caused by squash vein yellowing virus infection. Photo: S. Adkins, USDA-ARS.
Aphid-transmitted Virus
Cucurbit aphid-borne yellows virus (CABYV; genus Polerovirus) is transmitted by several aphid species, including the melon aphid, Aphis gossypii (Figure 12). Aphids that feed on and acquire CABYV from infected hosts can transmit the virus for the rest of their lives. Cucumbers, melons, squashes, watermelons, and other cucurbits can become infected with CABYV, but the virus can also infect a number of other hosts, including beets, lettuce, and several species of weeds, from which the virus can be transmitted to cucurbits. In the U.S., CABYV is known to occur in cucurbits in California and has also been reported in Alabama and Texas (Ali et al., 2012; Keinath, et al., 2017).
Symptoms: Plants infected with CABYV initially exhibit a green spotting or mottle similar to that observed for CYSDV, which develops into an interveinal chlorosis or yellowing on older leaves while most major veins remain green (Figure 13). These symptoms are also very similar to those induced by nutrient deficiencies, which can complicate diagnosis of infected plants. Symptomatic leaves are often brittle and thicker than symptomless leaves. The severity and extent of symptoms depends on the season, time of infection, and cultivar. Fruit quality on plants infected with CABYV does not appear to be affected, but vine growth and fruit quantity may be reduced.
Management: resistance and vector management
Symptoms similar to those caused by the viruses described above may also occur with other diseases or disorders. For example, cucurbits with cucurbit yellow vine disease (Figure 14), caused by the bacterium Serratia marcescens, or Fusarium wilt, caused by the fungus Fusarium oxysporum, may also exhibit a foliar chlorosis. Injury from some herbicides or various nutritional deficiencies, such as magnesium deficiency, may also cause foliar chlorosis.

Figure 12. Melon aphid (Aphis gossypii) adult and nymphs. Photo: M. B. Layton, Mississippi State University Extension Service.

Figure 13. Symptoms on bottle gourd (Lagenaria sp.) vines caused by cucurbit aphid borne yellows virus. Advanced symptom development is evident with yellowing between leaf veins and spread toward the ends of many of the vines. Photo: W. M. Wintermantel, USDA-ARS.

Figure 14. Symptoms of the bacterial disease cucurbit yellow vine disease in a watermelon vine. Photo: E. Sikora, Auburn University, Bugwood.org.
Diagnosis
Because similar symptoms are produced by a number of viruses, other pathogens, and abiotic factors, and because some of these viruses may occur in mixed infections (multiple viruses infecting the same plant), symptoms alone cannot be used to make an accurate diagnosis. Molecular laboratory tests are necessary in order to confirm that a symptomatic plant is infected with a virus and to specifically identify the virus or viruses causing the infection. However, not all diagnostic laboratories have the ability to perform the tests necessary to identify these yellowing viruses. If you suspect that your crop may be infected by one of these viruses, please contact your local diagnostic laboratory and/or your local Extension vegetable specialist before sending samples for disease identification.
When sending samples, it is important to provide detailed information about the symptoms, crop, and production practices as this information can provide relevant clues that help during the diagnostic process. Submitting digital photos along with the sample can also be useful. Because the described viruses are all spread by insect vectors, the presence of current or past infestations of insects, particularly aphids and whiteflies, should also be included.
Management
Biological Management
Plant varieties of cucurbit crops that have resistance to viruses or their insect vectors. Resistance to some of the previously described viruses has been identified in various cucurbit crops, but commercial varieties with resistance are not yet available for all host plants and viruses. Commercial melon varieties with resistance to the melon aphid are available and can delay the spread of CABYV. Resistance to CuLCrV is available in some varieties of cucumber and melon. Sources of resistance to CYSDV have been identified in melons and are being advanced into commercial varieties (McCreight et al., 2017), but these are not yet available. Similarly, a single source of resistance to CCYV has been identified in melons but is not yet available in commercial varieties.
Apply biological insecticides to manage insect vectors. Biological insecticides labeled for use against aphids and whiteflies are available, and some are approved for use in organic production. These are generally more effective in greenhouse settings, but they may also help reduce whitefly or aphid pressure in fields. A list of biological insecticides labeled for use against various insects in commercial production is available in the latest edition of the Southeastern U.S. Vegetable Crop Handbook (available online at www.vegcrophandbook.com). Visit www.omri.org for a complete list of products approved by the Organic Materials Review Institute for use in organic production. Always check with your organic certifier before purchasing and applying a product.
Cultural Management
Use plastic mulch or row covers. Plastic and/or silver reflective mulch (Figure 15) may reduce whitefly pressure and consequently delay the spread of some viruses. However, without eliminating whiteflies, you cannot entirely prevent virus infection.
Row covers can also be used to prevent exposure of young plants to whiteflies and slow the spread of disease. These can be removed as plants expand.
Monitor production fields or enclosed structures throughout the season for insect vectors. Yellow sticky traps can be used to monitor for aphids and whiteflies in production fields and enclosed structures. When populations of these insects are detected at target levels, make insecticide applications to reduce insect populations and limit potential for virus infection of plants.
Use insect-proof mesh on greenhouse openings. Adding insect-proof mesh on greenhouse openings can help keep potential vectors from entering and establishing populations in greenhouses. Insects that enter greenhouses may already be carrying viruses that can infect plants. A virus present in only a few plants in a greenhouse may not seem to be a problem. However, a virus can quickly spread if even just a few of the insect vectors find their way into the greenhouse and become established. As noted above, many of these viruses have long latent periods, so infection can develop even 3 or 4 weeks after the insect population has been eliminated.

Figure 15. Squash grown on reflective mulch. Photos: G. Holmes, Strawberry Center, Cal Poly San Luis Obispo, Bugwood.org
Sanitation
Plant transplants free of viruses and insect vectors. Transplants showing symptoms of virus infection should be destroyed. Planting infected transplants or transplants carrying insects may introduce viruses and/or their insect vectors into a field or enclosed structure. Under favorable conditions—when a virus and its insect vector both occur in the presence of susceptible hosts—the virus can spread rapidly and possibly become established in the area.
Remove diseased plants. Scout crops regularly for signs and symptoms of disease/virus infection. If infection is limited, immediately remove and destroy plants suspected of being infected with a virus. Virus-infected plants in a field or enclosed structure may serve as a source of the virus, which can be acquired by insect vectors and spread to new plants. As previously discussed, many yellowing viruses have a long latent period. If whiteflies were present in a nursery facility, there is the potential that transplants may be infected but not showing symptoms. Such plants should be monitored carefully for signs of virus infection.
Remove weed and volunteer cucurbit hosts. In areas that experience mild winters, volunteer cucurbits, previously infected crops, and weeds may survive the winter, and virus-infected plants may serve as a source from which insect vectors acquire a virus for transmission to crop plants. Removing previously infected crops, known weed hosts, and volunteer cucurbits can help to reduce or remove the source of one or more of these viruses, as well as plants on which their insect vectors can propagate.
Chemical Management
There are no chemical management methods that will eliminate viruses.
Apply insecticides to manage insect vectors. Insecticides can be an effective means of managing insect vectors, including whiteflies and aphids. This can reduce insect-feeding damage to cucurbit crops and the threat of virus transmission. Reducing the number of whiteflies can slow the rate at which plants become infected by reducing the load of virus being delivered. It is critical that insecticide treatments be rotated to prevent the buildup of insecticide resistance within insect populations.
A list of insecticides labeled for use against various insects in cucurbits grown for commercial production as well as an efficacy table of those insecticides against certain insects can be found in the latest edition of the Southeastern U.S. Vegetable Crop Handbook. Please be aware that not all products are registered for use in every state. To determine if a product is registered for use in your state, visit one of the online pesticide label databases that provide state registration information. A list of available databases is available in the Southeastern U.S. Vegetable Crop Handbook.
When using pesticides, remember: the label is the law. You must completely read product labels before use, and you must follow the label.
Survey for Whitefly-transmitted and Yellowing Viruses in Watermelon and Other Cucurbit Crops in Alabama, Louisiana, and Mississippi
It is not presently known if the described viruses are present in Alabama, Louisiana, and Mississippi. The most recent virus survey in these states occurred in 2010 and 2011. Of the viruses described in this publication, only CABYV was among the viruses tested in that survey, and it was only identified in two (out of 13) samples from Alabama (Ali et al., 2012).
In 2020, scientists will conduct a survey of watermelon and some other cucurbit fields in these states to look for these yellowing viruses and their whitefly vectors. Samples of cucurbit plants showing symptoms of yellowing viruses and of whiteflies present in fields will be collected and tested to determine the presence of target viruses and whitefly biotype. Results from this survey will provide valuable information as to the occurrence of these viruses in these states and will be used to update best management practices for watermelon and cucurbit production. These efforts are part of the project “Survey of whitefly-transmitted viruses in watermelon and other cucurbits in Alabama, Louisiana, and Mississippi,” supported by funding from the National Watermelon Association. For more information on this project and to volunteer to be a grower-collaborator, please visit http://msuext.ms/h27md.
References
Adkins, S., McCollum, T. G., Albano, J. P., Kousik, C. S., Baker, C. A., Webster, C. G., Roberts, P. D., Webb, S. E., and Turechek, W. W. 2013. Physiological effects of Squash vein yellowing virus infection on watermelon. Plant Disease 97:1137–1148.
Ali, A., Abdalla, O., Bruton, B., Fish, W., Sikora, E., Zhang, S., and Taylor, M. 2012. Occurrence of viruses infecting watermelon, other cucurbits, and weeds in the parts of southern United States. Online. Plant Health Progress doi:10.1094/PHP-2012-0824-01-RS.
Keinath, A. P., Wintermantel, W. M., and Zitter, T. A. (eds). 2017. Compendium of Cucurbit Diseases and Pests, second edition. American Phytopathological Society Press, St. Paul, MN. 220 pages.
Kousik, C. S. and Adkins, S. 2020. Detection of cucurbit yellow stunting disorder virus infecting watermelon in South Carolina. Plant Health Progress Online. doi:https://doi.org/10.1094/PHP-03-20-0016-BR.
McCreight, J. D., Liu, H.-Y., and Turini, T. A. 2008. Genetic resistance to Cucurbit leaf crumple virus in melon. HortScience 43:122–126.
McCreight, J. D., Wintermantel, W. M., Natwick, E. T., Sinclair, J. W., Crosby, K. M., and Gómez-Guillamón, M. L. 2017. Recessive resistance to Cucurbit yellow stunting disorder virus in melon TGR 1551. Acta Horticulturae 1151:101–107.
Okuda, M., Okazaki, S., Yamasaki, S., Okuda, S., and Sugiyama, M. 2010. Host range and complete genome sequence of Cucurbit chlorotic yellows virus, a new member of the genus Crinivirus. Phytopathology 100:560–566.
Wintermantel, W. M., Hladky, L. L., Cortez, A. A., and Natwick, E. T. 2009. A new expanded host range of Cucurbit yellow stunting disorder virus includes three agricultural crops. Plant Disease 93:685–690.
Wintermantel, W. M., Jenkins Hladky, L. L., Fashing, P., Ando, K., and McCreight, J. D. 2019. First report of cucurbit chlorotic yellows virus infecting melon in the New World. Plant Disease 103(4):778.
Additional Resources
Southeastern U.S. Vegetable Crop Handbook, available at http://www.vegcrophandbook.com.
Diagnostic Laboratories in Alabama, Louisiana, and Mississippi
Auburn Plant Diagnostic Lab: https://offices.aces.edu/plantlabauburn/
Louisiana State University Agricultural Center Plant Diagnostic Center: www.lsuagcenter.com/plantdiagnostics
Mississippi State University Extension Plant Diagnostic Lab: http://extension.msstate.edu/lab
Glossary of Plant Pathology and Entomology Terms
abiotic – describes an agent or factor that is not living
biotype – a type of subgroup within some insect species such as whiteflies; individuals within a designated biotype share specific biological characteristics that are different from those of individuals in another biotype
chlorosis (adj. chlorotic) – a type of symptom; pale green or yellow discoloration of normally green tissues
criniviruses – a group of related whitefly-transmitted viruses in the genus Crinivirus known for causing yellowing symptoms on infected plants
diagnosis – the accurate identification of the cause of a disease
disease – an abnormality in a plant caused by infection with a biotic (living) agent
disorder – an abnormality in a plant caused by an abiotic (non-living) factor
enclosed structure – any structure that is covered in whole or in part by a non-porous covering; enclosed structures include greenhouses and high tunnels
latent period – the time between when a plant becomes infected and when symptoms develop
mosaic – a type of symptom usually caused by virus infection; a pattern of intermingled, distinct patches of alternating shades, usually green or yellow, on a leaf; a mosaic is similar to but generally involves more distinct separation of color shades than a mottle
mottle – a type of symptom usually caused by virus infection; a pattern of less distinct or spotty patches of color on leaves or other plant parts; a mottle is similar to but may be less extensive than a mosaic
necrosis (adj. necrotic) – a type of symptom; the death of plant tissue; necrotic tissue is often brown
pathogen – a disease-causing organism/agent; common groups of pathogens include bacteria, fungi, nematodes, oomycetes, and viruses
symptom – an indication of disease; the plant’s reaction to infection by a pathogen; examples: chlorosis, lesions, mosaic, mottle, wilting
vector – an insect that is capable of acquiring a virus from an infected plant and transmitting it to a non-infected plant
Acknowledgement
This publication was made possible through the project “Survey of whitefly-transmitted viruses in watermelon and other cucurbits in Alabama, Louisiana, and Mississippi,” supported by funding from the National Watermelon Association. Any opinions or recommendations expressed in this publication do not necessarily reflect the views of the National Watermelon Association.

Publication 3439 (POD-05-23)
Prepared by Rebecca A. Melanson, PhD, Assistant Extension Professor, Plant Pathology, Mississippi State University Central Mississippi Research and Extension Center; William M. Wintermantel, PhD, Research Plant Pathologist, USDA-ARS; Edward J. Sikora, PhD, Professor and Extension Plant Pathologist, Alabama Cooperative Extension System, Auburn University; and Raghuwinder Singh, DPM, Associate Professor and Director of the Plant Diagnostic Center, Louisiana State University Agricultural Center. Reviewed by Clarissa Balbalian, Diagnostic Laboratory Manager, MSU Extension; Sead Sabanadzovic, PhD, Professor, Plant Pathology, Mississippi State University; Blake Layton, PhD, Extension Professor, Entomology, MSU Extension; and Heath Steede, George County ANR Extension Agent, MSU Extension.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.


